Publications after joining BITS-Pilani Goa (2022 – Present)
(# = equal contribution; * = corresponding author; IF = Impact Factor)
Book Edited
Dr. Kushal Sengupta, Dr. Sudipta Chatterjee, Dr. Kingshuk Dutta. Oxygen Reduction Reaction:
Fundamentals, Materials, and Applications, 1st Edition - June 14, 2022 by Elsevier. eBook ISBN: 9780323907200; Paperback ISBN: 9780323885089.
Book Chapter
- Md E. Ahmed, S. Chattopadhyay, S. Chatterjee, K. Sengupta (2022); ‘Chapter 12 – Oxygen Reduction Reaction in Enzymatic Biofuel Cells’, Editor(s): K. Sengupta, S. Chatterjee, K. Dutta, Oxygen Reduction Reaction: Fundamentals, Materials, and Applications by Elsevier, Pages 427-466, ISBN 9780323885089. (DOI: https://doi.org/10.1016/B978-0-323-88508-9.00008-2)
- K. Mittra, S. Samanta, A. Singha, K. Sengupta, S. Chatterjee (2022); ‘Chapter 2 – Oxygen Reduction Reaction by Metalloporphyrins’, Editor(s): K. Sengupta, S. Chatterjee, K. Dutta, Oxygen Reduction Reaction: Fundamentals, Materials, and Applications by Elsevier, Pages 45-77, ISBN 9780323885089. (DOI: https://doi.org/10.1016/B978-0-323-88508-9.00003-3)
Journal Publications
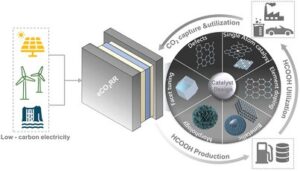
4. K. Peramaiah, M. Yi, I. Dutta, S. Chatterjee, H. Zhang, Z. Lai, K.-W. Huang*; Catalyst Design and Engineering for CO2-to-Formic Acid Electrosynthesis for a Low-Carbon Economy; Adv. Mater., 2024, 2404980. (Review) [Link] [IF = 27.4]
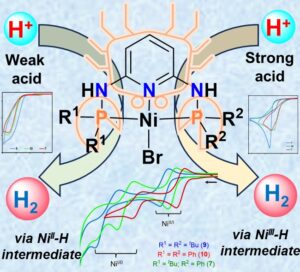
3.S. Chatterjee,#* I. Dutta,# B. Dereli,# P. Chakraborty, K. Peramaiah, N. Gupta, L. Cavallo,* K.-W. Huang*; Electrocatalytic Hydrogen Generation by Ni-PN3P Pincer Complexes: Role of Phosphorus Substituents in Tuning the Reactivity; Chem. Asian J., 2024, e202400690. [Link] [IF = 3.5]
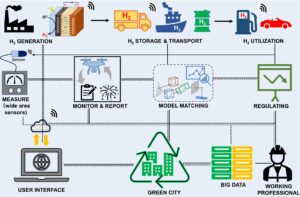
2. Y. Cai#, S. Chatterjee#, K. N. Salama, L. -J. Li, K. -W. Huang*; Sensing fugitive hydrogen emissions;Nat. Rev. Electr. Eng., 2024, 1, 201 - 211 (Comment) [Link] [IF = N/A]
 1.
1.
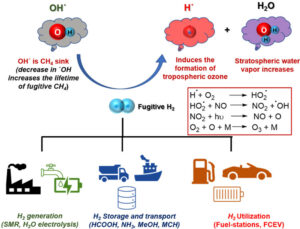
1.I. Dutta#, R. K. Parsapur#, S. Chatterjee, A. M. Hengne, D. Tan, K. Peramaiah, T. I. Solling, O. J. Nielsen, K.-W. Huang*; The Role of Fugitive Hydrogen Emissions in Selecting Hydrogen Carriers; ACS Energy Lett., 2023, 8, 3251 – 3257. (Viewpoint) [Link] [IF = 23.99]
Publications before joining BITS-Pilani Goa
Book Chapter
2. Chatterjee, I. Dutta, K.-W. Huang* (2021); ‘Chapter 8 – Power to Formic Acid’, in G. Spazzafumo (ed.) Power to Fuel: How to Speed up a Hydrogen Economy by Elsevier, Pages 169 – 210. (DOI: https://doi.org/10.1016/B978-0-12-822813-5.00006-0 ).
1. Chatterjee*, K. Sengupta (2020); ‘Chapter 6 – Carbon-Based Electrodes for Direct Methanol Fuel Cell’, in K. Dutta (ed.) Direct Methanol Fuel Cell Technology by Elsevier, Pages 135-176. (DOI: https://doi.org/10.1016/B978-0-12-819158-3.00006-9 ).
Publications From Post-doc
33. I. Dutta#, Chatterjee#, H. Cheng#, R. K. Parsapur#, Z. Liu,* Z. Li, E. Ye, H. Kawanami,* J. S. C. Low, Z. Lai, X. J. Loh,* K.-W. Huang*; Formic Acid to Power towards Low-Carbon Economy; Adv. Energy Mater., 2022, 2103799. (Perspective) [Link] [IF = 29.69]
32. Chatterjee#, R. K. Parsapur#, K.-W. Huang*; Limitations of Ammonia as a Hydrogen Energy Carrier for the Transportation Sector; ACS Energy Lett., 2021, 6, 4390 – 4394. (Viewpoint) [Link] [IF = 23.99]
31. Chatterjee#, I. Dutta#, Y. Lum, Z. Lai, K-W Huang*; Enabling Storage and Utilization of Low-Carbon Electricity: Power to Formic Acid; Energy Environ. Sci., 2021, 14, 1194 – 1246. (Review) [Link] [IF = 39.71]
30. P. K. Das, S. Bhunia, P. Chakraborty, Chatterjee, A. Rana, K. Peramaiah, M. M. Alsabban, I. Dutta, A. Dey*, and K-W. Huang*; Electrocatalytic Water Oxidation by a Phosphorus–Nitrogen O═PN3-Pincer Cobalt Complex; Inorg. Chem. 2021, 60, 614-622. [Link] [IF = 5.43]
29. R. K. Parsapur, Chatterjee, K.-W. Huang*; The Insignificant Role of Dry Reforming of Methane in CO2 Emission Relief; ACS Energy Lett., 2020, 5, 2881. (Viewpoint) [Link] [IF = 23.99]
28. Chatterjee, K.-W. Huang*; Unrealistic Energy and Materials Requirement for Direct Air Capture in Deep Mitigation Pathways; Nat. Commun., 2020, 11, 3287. (Matters Arising) [Link] [IF = 17.69]
27. I. M. DiMucci, J. T. Lukens, Chatterjee, K. M. Carsch, C. J. Titus, S. J. Lee, D. Nordlund, T. A. Betley, S. N. MacMillan, K. M. Lancaster*; The Myth of d8 Copper(III); J. Am. Chem. Soc., 2019, 141, 11508-11520. (Highlighted in Nature Reviews Chemistry) [Link] [IF = 16.38]
26. P. L. Dunn#, Chatterjee#, S. M. MacMillan, A. J. Pearce, K. M. Lancaster*, I. A. Tonks*; The 4-Electron Cleavage of a N═N Double Bond by a Trimetallic TiNi2 Complex; Inorg. Chem. 2019, 58, 11762-11772. [Link] [IF = 5.43]
25. T. Moore#, S. Chaterjee#, M. Tarrago#, L. J. Clouston, S. Sproules, E. Bill, V. Bernales, L. Gagliardi, S. Ye*, K. Lancaster*, C. C. Lu*; Enhanced Fe-centered Redox Flexibility in Fe-Ti Heterobimetallic Complexes; Inorg. Chem. 2019, 58, 6199-6214. [Link] [IF = 5.43]
Publications From Ph.D.
24. A. Sarkar, K. Sengupta, Chatterjee, M. Seal, P. Faller, S. Ghosh Dey, A. Dey*; Metal Binding to Aβ Peptides Inhibits Interaction with Cytochrome c: Insights from Abiological Constructs; ACS Omega, 2018, 3, 13994-14003 (selected as ‘ACS Authors’ Choice’). [Link] [IF = 4.13]
23. Chattopadhyay, A. Sarkar, S. Chatterjee, A. Dey*; Functional adlayers on Au electrodes: some recent applications in hydrogen evolution and oxygen reduction;J. Mater. Chem. A, 2018, 6, 1323-1339. (Invited Review) [Link] [IF = 14.51]
22. Chatterjee, K. Sengupta, B. Mondal, S. Dey, A. Dey*; Factors Determining the Rate and Selectivity of 4e–/4H+ Electrocatalytic Reduction of Dioxygen by Iron Porphyrin Complexes;Acc. Chem. Res., 2017, 50, 1744-1753. [Link] [IF = 24.46]
21. Dey, B. Mondal, S. Chatterjee, A. Rana, Sk Amanullah, A. Dey*; Molecular electrocatalysts for the oxygen reduction reaction;Nat. Rev. Chem., 2017, 1, 0098. (Invited Review) [Link] [IF = 34.57]
20. Sengupta, S. Chatterjee, A. Dey*; In Situ Mechanistic Investigation of O2 Reduction by Iron Porphyrin Electrocatalysts Using Surface-Enhanced Resonance Raman Spectroscopy Coupled to Rotating Disk Electrode (SERRS-RDE) Setup; ACS Catal.,2016, 6, 6838-6852. (Invited Perspective) [Link] [IF = 13.70]
19. Chatterjee#, K. Sengupta#, S. Bandyopadhyay, A. Dey*; Ammonium Tetrathiomolybdate as a novel electrode material for Convenient Tuning of Kinetics of Electrochemical O2 Reduction by Iron Porphyrin Catalysts; J. Mater. Chem. A,2016, 4, 6819 (published in a themed issue of Emerging Investigators 2016: Novel design strategies for new functional materials). [Link] [IF = 14.51]
18. Sengupta#, S. Chatterjee#, A. Dey*; Catalytic H2O2 disproportionation and electrocatalytic O2 reduction by functional mimic of Heme Catalase: direct observation of Compound 0 and Compound I in-situ; ACS Catal.,2016, 6, 1382 (selected as ‘ACS Editors’ Choice’). [Link] [IF = 13.70]
17. Mittra, K. Sengupta, A. Rana, S. Bandyopadhyay, S. Chatterjee, S. Samanta, A. Dey*; Second Sphere Control of Spin State: Differential Tuning of Axial Ligand Bonds in Ferric Porphyrin Complexes by Hydrogen Bonding; J. Inorg. Biochem. 2016, 155, 82. [Link] [IF = 4.33]
16. Chatterjee, K. Sengupta, S. Hematian, K. D. Karlin, A. Dey*; Electrocatalytic O2 Reduction by Synthetic Cytochrome C Oxidase Mimics: Identification of a ‘Bridging Peroxo’ Intermediate Involved in Facile 4e-/4H+ O2 Reduction; J. Am. Chem. Soc., 2015, 137, 12897. [Link] [IF = 16.38]
15. Chatterjee, K. Sengupta, S. Samanta, P. K. Das, A. Dey*; Concerted Proton-Electron Transfer in Electrocatalytic O2 Reduction by Iron Porphyrin Complexes: Axial Ligands Tuning H/D Isotope Effect; Inorg. Chem.,2015, 54, 2383. [Link] [IF = 5.43]
14. S. Samanta, P. K. Das, Chatterjee, A. Dey*; Effect of axial ligands on electronic structure and O2 reduction by iron porphyrin complexes: Towards a quantitative understanding of the "push effect"; J. Porphyrins Phthalocyanines, 2015, 19, 92 (Invited Review). [Link] [IF = 1.91]
13. K. Sengupta#, Chatterjee#, D. Pramanik, S. Ghosh Dey*, A. Dey*; Self-assembly of stable oligomeric and fibrillar aggregates of Aβ peptides relevant to Alzheimer's disease: morphology dependent Cu/heme toxicity and inhibition of PROS generation; Dalton Trans.,2014, 43, 13377-13383. [Link] [IF = 4.57]
12. S. Mukherjee, S. Bandyopadhyay, Chatterjee, A. Dey*; Electrocatalytic O2 reduction by a monolayer of hemin: the role of pKa of distal and proximal oxygen of a FeIII-OOH species in determining reactivity; Chem. Commun., 2014, 50, 12304. [Link] [IF = 6.22]
11. K. Sengupta, Chatterjee, S. Mukherjee, S. Ghosh Dey*, A. Dey*; Heme bound amylin self-assembled monolayers on an Au electrode: an efficient bio-electrode for O2 reduction to H2O; Chem. Commun., 2014, 50, 3806. [Link] [IF = 6.22]
10. Mittra, S. Chatterjee, S. Samanta, A. Dey*; Selective 4e−/4H+ O2 Reduction by an Iron(tetraferrocenyl)Porphyrin Complex: From Proton Transfer Followed by Electron Transfer in Organic Solvent to Proton Coupled Electron Transfer in Aqueous Medium; Inorg. Chem., 2013, 52, 14317-14325. [Link] [IF = 5.43]
9. Chatterjee, K. Sengupta, S. Dey, A. Dey*; Ammonium Tetrathiomolybdate: A Versatile Catalyst for Hydrogen Evolution Reaction from Water under Ambient and Hostile Conditions; Inorg. Chem.,2013, 52, 14168-14177. [Link] [IF = 5.43]
8. S. Samanta, P. K. Das, Chatterjee, K. Sengupta, B. Mondal, A. Dey*; O2 Reduction Reaction by Biologically Relevant Anionic Ligand Bound Iron Porphyrin Complexes; Inorg. Chem., 2013, 52, 12963-12971. [Link] [IF = 5.43]
7. Chatterjee, K. Sengupta, S. Samanta, P. K. Das, A. Dey*; Electrocatalytic O2 reduction reaction by synthetic analogues of cytochrome P450 and myoglobin: in-situ resonance Raman and dynamic electrochemistry investigations;Inorg. Chem., 2013, 52, 9897-9907. [Link] [IF = 5.43]
6. Sengupta, S. Chatterjee, S. Samanta, A. Dey*; Direct observation of intermediates formed during steady-state electrocatalytic O2 reduction by iron porphyrins; Proc. Nat. Acad. Sci. U.S.A., 2013, 110, 8431 (Highlighted in Nature India-2013). [Link][IF = 12.78]
5. Sengupta, S. Chatterjee, S. Samanta, S. Bandyopadhyay, A. Dey*; Resonance Raman and Electrocatalytic Behavior of Thiolate and Imidazole Bound Iron Porphyrin Complexes on Self Assembled Monolayers: Functional Modeling of Cytochrome P450; Inorg. Chem., 2013, 52, 2000–2014 (highlighted in the virtual issue “Models of Metalloenzymes”) [Link] [IF = 5.43]
4. S. Samanta#, K. Mittra#, Sengupta, S. Chatterjee, A. Dey*; Second Sphere Control of Redox Catalysis: Selective Reduction of O2 to O2- or H2O by an Iron Porphyrin Catalyst; Inorg. Chem., 2013, 52, 1443–1453. [Link] [IF = 5.43]
3. Chatterjee, K. Sengupta, S. Bhattacharyya, A. Nandi, S. Samanta, K. Mittra, A. Dey*; Photophysical and ligand binding studies of metalloporphyrins bearing hydrophilic distal superstructure; J. Porphyrins Phthalocyanines, 2013, 17, 210-219 (Invited Article). [Link] [IF = 1.91]
2. K. Das, S. Chatterjee, S. Samanta, A. Dey*; EPR, Resonance Raman, and DFT Calculations on Thiolate- and Imidazole-Bound Iron(III) Porphyrin Complexes: Role of the Axial Ligand in Tuning the Electronic Structure; Inorg. Chem., 2012, 51, 10704. [Link] [IF = 5.43]
1. K. Mittra, Chatterjee, S. Samanta, K. Sengupta, H. Bhattacharjee, A. Dey*; A hydrogen bond scaffold supported synthetic heme FeIII-O2- adduct; Chem. Commun., 2012, 48, 10535-10537. [Link] [IF = 6.22]


 An Institute of Eminence
An Institute of Eminence



 1.
1.






